
Clinical Data
About the inFoods IBS® Clinical Study
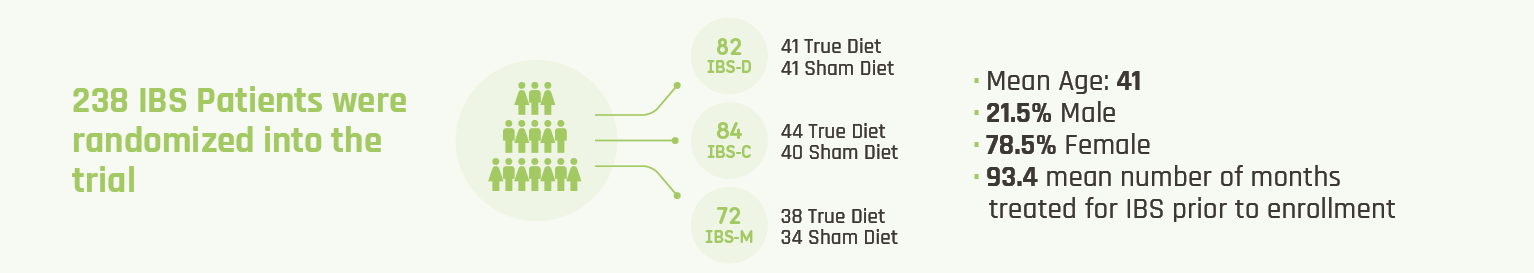
In a Prospective, Multi-Center, Double-Blind, Placebo-Controlled Study,
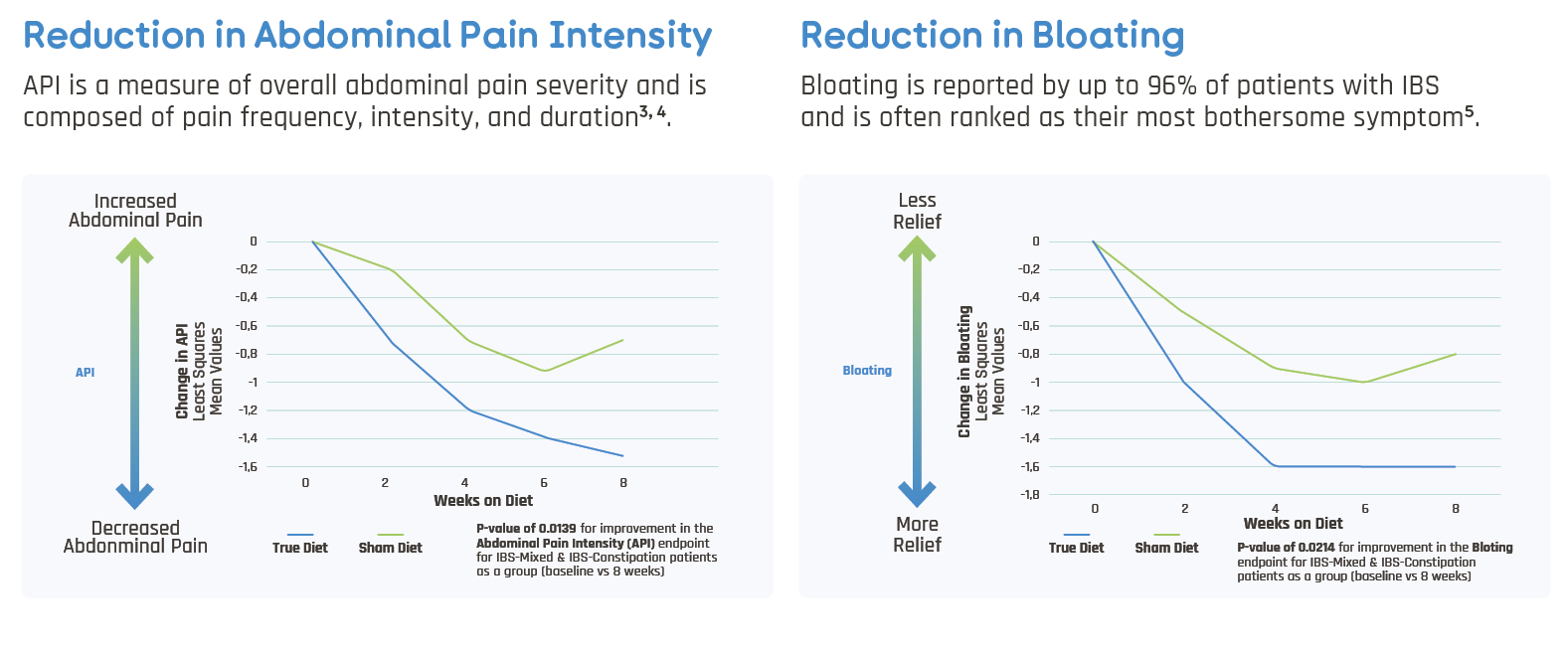
an inFoods® IBS elimination diet demonstrated a remarkable and statistically significant reduction in IBS symptoms over an 8-week period
- Study Sites: Mayo Clinic, Harvard Beth-Israel, University of Michigan, University of Texas Houston
- Study Design: 8-week, prospective, multi-center, double-blinded, placebo-controlled, randomized
- Treatment Arm: True Diet - Elimination of positive foods (elevated levels of IgG) from the diet of IBS patients
- Placebo Arm: Sham Diet – Elimination of non-positive foods (normal levels of IgG) from the diet of IBS patients
Endpoint Determination Study for An Antibody Guided Dietary Restriction Trial Using Biomerica inFoods® IBS Test in Patients With a Previous Diagnosis of Irritable Bowel Syndrome (IBS) (NCT03459482)
Investigators

William Chey, MD, AGAF, FACG, FACP
Principal Investigator
University of Michigan – Ann Arbor
Director of the Digestive Diseases Center
Co-Author of ACG Guidelines

Anthony Lembo, MD, FACG
Principal Investigator
Harvard - Beth Israel Deaconess Medical Center
Director, Center for GI Motility and Functional Bowel Disorders

Tisha Lunsford, MD
Mayo Clinic
Director of the Motility Interest Group

Brian Lacy, MD, PhD, FACG
Mayo Clinic
Former Co-Editor in Chief of the American Journal of Gastroenterology
Co-Author of ACG Guidelines

Eamonn Quigley, MD, MACG
Chief, Division of Gastroenterology and Hepatology at Houston Methodist

Brooks Cash, MD, AGAF, FACG, FACP, FASGE
University of Texas Health Science Center at Houston,
Chief of Gastroenterology

What if the results from a simple blood test could decipher which foods trigger IBS symptoms?

